CANNIZARO REACTION
Ever wondered how it would be to learn few important reactions in a very less time?? Here you go guys about cannizaro reaction, its types and mechanism
i)
This is an example of
intramolecular reaction
ii)
This reaction is redox and
dispropornation reaction
2. Intermolecular condensation:
i) NaOH will attack on carbon of
formaldehyde because, carbon of formic acid is more acidic
ii) Due to +R effect of ph-
Trick
In cannizzarro reaction, one
compound gets oxidizes & the other gets reduced.
Generally the compound with
more acidic carbon gets oxidized & the one with less acidic gets reduced.
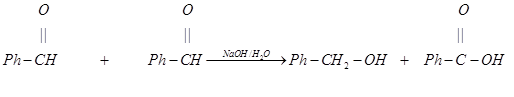
ØIn a mixture of formaldehyde &
phenyl aldehyde, when formaldehyde reacts with phenyl aldehyde reacts with
phenyl aldehyde then it is called cross cannizzaro reaction
When
Reacts
with themselves then it is known as ‘self-cannizzarro reaction’
(one
part will get oxidized and other gets reduced)
So
4 possible products by self & cross cannizzaro reaction
Self
cannizzaro & intramolecular are both redox & disproportanation whereas
cross Cannizzaro is an only redox reaction
Trick
1)
In condensation reactions a new
carbon-carbon bond formation takes place while in case of cannizzaro reaction
oxidation- reduction takes place
2)
Cannizzaro reaction => possible only for Benzaldehydes & formaldehyde
(those aldehydes which do not have alpha-hydrogen)
3)
Aldol condensation => possible only
for aldehydes having at least on alpha- hydrogen
4)
Cross aldol condensation => possible for 2
different aldehydes having at least one alpha-hydrogen
[note: exception is
Benzaldehyde & other aldehyde having at least one alpha-hydrogen]











No comments:
Post a Comment